What is limestone?
Rainwater, originally pure, becomes hard as it passes through soils rich in limestone rocks. By dissolving minerals, it becomes charged with calcium ions (Ca²⁺) and magnesium ions (Mg²⁺), which determine its hardness. The calcium content of your water, its hardness, will therefore be primarily determined by the geology of your region.
The more minerals the water contains, the more it is considered "hard", and the more it tends to leave limescale deposits in pipes and appliances.
Hardness is expressed in °f (French degree)corresponding to 10 mg calcium carbonate (CaCO₃) / liter.
The disadvantages of hard water are :
- Scale formation: When heated, calcium and magnesium crystallize as limescale deposits in pipes, water heaters, faucets, and household appliances.
- Reduced energy efficiency: A thin layer of limescale in a water heater or boiler can increase energy consumption, as heat exchange becomes less efficient. Heating, therefore becomes more expensive.
- Dry skin and hair: Hard water dries out the skin and hair, as it reduces the effectiveness of soaps and shampoos.
- Stiff laundry: Limescale makes clothes feel stiffer after washing and reduces the effectiveness of laundry detergents.
To avoid these drawbacks, installing a water softener is an effective solution.

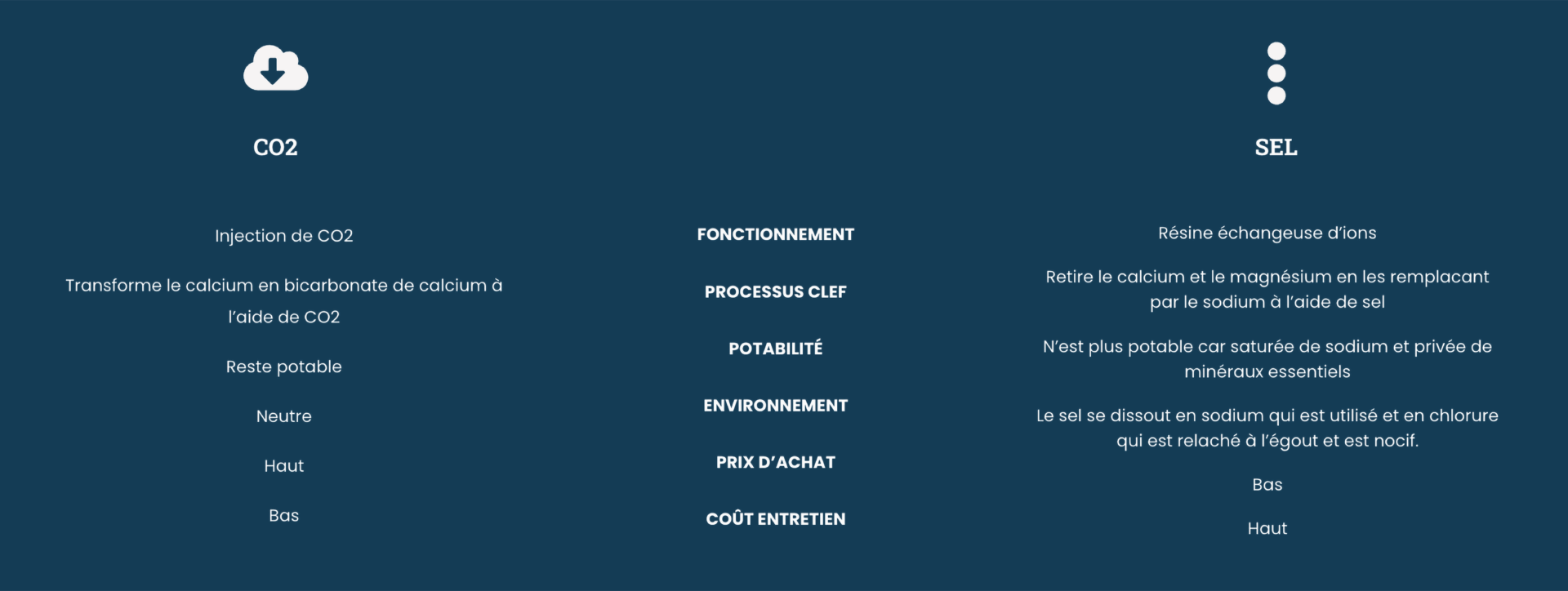
Is an ecological and economical salt water softener possible?
Aquacycle fights for a pure water and healthy for you and the environment.
Softening water with salt releases a harmful concentration of chloride directly down the drain. However, not everyone can afford to invest in a CO2.
That’s why Aquacycle offers an intelligent salt-based water softener:
- Statistical valve: detects the saturation level to use only the strictly necessary amount of water and salt, in order to limit the impact on your wallet and the environment.
- Amberlight resin: has an ion exchange capacity 30% higher than standard resins, requiring less water during rinsing.

4 questions to choose the size of your salt water softener
1. Determine your water hardness
Water hardness, expressed in French degrees (°f), indicates the concentration of calcium and magnesium ions responsible for limescale formation.
You can obtain this information by consulting your water bill, your water distributor's website or by using a hardness test kit available from Aquacycle.
2. Evaluate your water consumption
Estimate the amount of water used by your household over a 10-day period.
Example: your annual consumption is 120 m³, which corresponds to approximately 3.28 m³ over 10 days (120 m³ / 365 days × 10).
3. Calculate the required softener capacity
Multiply your 10-day consumption by your water hardness to obtain the required treatment capacity in °f.m³.
Example: for a consumption of 3.28 m³ and a hardness of 35 °f, the calculation would be: 3.28 m³ × 35 °f = 114.8 °f.m³.
4. Determine the volume of resin required
One liter of resin treats about 5 °f.m³ of water, divide the treatment capacity by this value.
In our example: 114.8 °f.m³ / 5 °f.m³/L = 22.96 L. It therefore makes sense to opt for a softener with a resin volume of at least 23 liters.
How does the installation work? 2 Options

1) Remote guided installation: free of charge
When making your purchase, select the option of self-installation. Your order will then be delivered to you, and you'll install it yourself, guided by the installation instructions and, if necessary, our remote video and audio support.

2) Home installation: paying
When you make your purchase, select the option for installation by one of our professionals. Your order will then be delivered to you, and we'll contact you to schedule a professional installation.
Tips & Tricks
Articles and other tools to help you form a critical opinion on the subject of water filtration.